
Chemistry, 22.08.2019 02:20 lisacarter0804
The boiling point of bromine is 59 °c. which of the following best predicts the boiling point of iodine monochloride, a polar compound?
higher than 59 °c because dipole-dipole interactions in iodine monochloride are stronger than dispersion forces in bromine.
lower than 59 °c because ionic bonding in bromine is stronger than covalent bonding in iodine monochloride.
lower than 59 °c because dipole-dipole interactions in iodine monochloride are weaker than in bromine.
higher than 59 °c because ionic bonding in iodine monochloride is stronger than h-bonding in bromine.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1
You know the right answer?
The boiling point of bromine is 59 °c. which of the following best predicts the boiling point of iod...
Questions

Mathematics, 05.10.2019 10:30

English, 05.10.2019 10:30

English, 05.10.2019 10:30


Chemistry, 05.10.2019 10:30


History, 05.10.2019 10:30






Mathematics, 05.10.2019 10:30



History, 05.10.2019 10:30


History, 05.10.2019 10:30

Mathematics, 05.10.2019 10:30

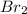 ) is a non-polar molecule due to the absence of a permanent dipole. The only force of attraction in non-polar molecules are the weak dispersion forces.
) is a non-polar molecule due to the absence of a permanent dipole. The only force of attraction in non-polar molecules are the weak dispersion forces. 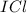 ) is a polar molecule which are held together dipole-dipole forces that are stronger than the London dispersion forces.
) is a polar molecule which are held together dipole-dipole forces that are stronger than the London dispersion forces. 

