
Chemistry, 21.08.2019 05:00 jessicasbss6840
[co(h2o)6]2+(aq) + 4cl-(aq) ⇌ [cocl4]2-(aq) + 6h2o(l)
concentrations at 25 degrees c
h+ 9.84368e-8
oh- 9.84368e-8
co (h2o)6^2 0.966539
cl- 1.86616
cocl4^2- 0.0334612
concentrations at 65 degree c
h+ 3.73844e-7
oh- 3.73844e-7
co (h2o)6^2 0.765375
cl- 1.06150
cocl4^2- 0.234625
a. write a k expression for this reaction (note that that liquid water shouldn’t be included in the k expression).
b. use the k expression and equilibrium concentrations on the left to determine the k value at 25 deg c. show all work for full credits.
c. what is the equilibrium constant k’ 65 degrees c?
d. compare the k’ constants; are the value difference agree or against with endo or exothermic determination?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
[co(h2o)6]2+(aq) + 4cl-(aq) ⇌ [cocl4]2-(aq) + 6h2o(l)
concentrations at 25 degrees c
h+...
concentrations at 25 degrees c
h+...
Questions


Chemistry, 09.02.2021 01:00

Chemistry, 09.02.2021 01:00


Mathematics, 09.02.2021 01:00


Physics, 09.02.2021 01:00

English, 09.02.2021 01:00

Mathematics, 09.02.2021 01:00




Physics, 09.02.2021 01:00







Mathematics, 09.02.2021 01:00

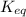 is given below.
is given below.![[Co(H_2O)_6]^{2+}(aq.)+4Cl^-(aq.)\rightleftharpoons [CoCl_4]^{2-}(aq.)+6H_2O(l)](/tpl/images/0183/8321/62682.png)
![K_{eq}=\frac{[CoCl_4]^{2-}}{[Co(H_2O)_6]^{2+}[Cl^-]^4}](/tpl/images/0183/8321/7356e.png) ......(1)
......(1)![[CoCl_4]^{2-}=0.0334612M](/tpl/images/0183/8321/e655c.png)
![[Co(H_2O)_6]^{2+}=0.966539M](/tpl/images/0183/8321/d26ec.png)
![[Cl^-]=1.86616M](/tpl/images/0183/8321/c7ee8.png)
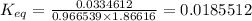
![[CoCl_4]^{2-}=0.234625M](/tpl/images/0183/8321/4e090.png)
![[Co(H_2O)_6]^{2+}=0.765375M](/tpl/images/0183/8321/d7c6a.png)
![[Cl^-]=1.06150M](/tpl/images/0183/8321/94289.png)
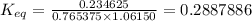
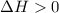 , which is positive
, which is positive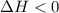 , which is negative
, which is negative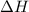 of the reaction, we use Van't Hoff's equation, which is:
of the reaction, we use Van't Hoff's equation, which is:![\ln(\frac{K_{65^oC}}{K_{25^oC}})=\frac{\Delta H}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0183/8321/25a2a.png)
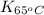 = equilibrium constant at 65°C = 0.2887886
= equilibrium constant at 65°C = 0.2887886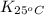 = equilibrium constant at 25°C = 0.0185512
= equilibrium constant at 25°C = 0.0185512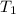 = initial temperature =
= initial temperature = ![25^oC=[25+2730]K=298K](/tpl/images/0183/8321/87ed3.png)
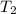 = final temperature =
= final temperature = ![65^oC=[65+2730]K=338K](/tpl/images/0183/8321/aec57.png)
![\ln(\frac{0.2887886}{0.0185512})=\frac{\Delta H}{8.314J/mol.K}[\frac{1}{298}-\frac{1}{338}]\\\\\Delta H=57471.26J/mol](/tpl/images/0183/8321/e16a7.png)


