
Consider the following reaction where kc = 9.52×10-2 at 350 k. ch4(g) + ccl4(g) 2ch2cl2(g)a reaction mixture was found to contain 2.18×10-2 moles of ch4(g), 3.79×10-2 moles of ccl4(g) and 1.09×10-2 moles of ch2cl2(g), in a 1.00 liter container. is the reaction at equilibrium? if not, what direction must it run in order to reach equilibrium? the reaction quotient, qc, equals .the reactiona. must run in the forward direction to reach equilibrium. b. must run in the reverse direction to reach equilibrium. c. is at equilibrium.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1


Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
Consider the following reaction where kc = 9.52×10-2 at 350 k. ch4(g) + ccl4(g) 2ch2cl2(g)a reaction...
Questions









Computers and Technology, 28.01.2020 05:31




Social Studies, 28.01.2020 05:31




Computers and Technology, 28.01.2020 05:31



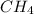 =
= 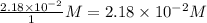
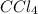 =
= 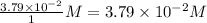
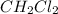 =
= 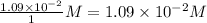
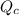 , for this reaction =
, for this reaction = ![\frac{[CH_{2}Cl_{2}]^{2}}{[CH_{4}][CCl_{4}]}](/tpl/images/0183/1287/5dd97.png)
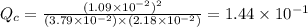
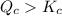 therefore reaction must run in reverse direction to reduce
therefore reaction must run in reverse direction to reduce 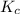 .
.

