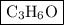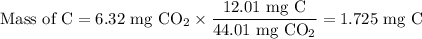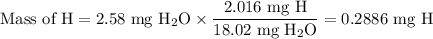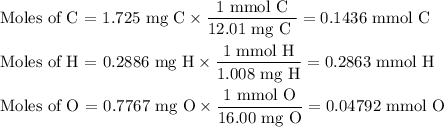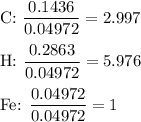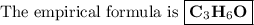Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
Combustion of 2.78 mg of ethyl butyrate produces 6.32 mg of co2 and 2.58 mg of h2o. what is the empi...
Questions







Spanish, 22.05.2021 03:20




Mathematics, 22.05.2021 03:20


Mathematics, 22.05.2021 03:20


English, 22.05.2021 03:20




Mathematics, 22.05.2021 03:20

Mathematics, 22.05.2021 03:20