
Chemistry, 20.08.2019 02:30 notseansafe
Consider the following equilibrium: co2(g) + c(graphite) 2co(g); δh = 172.5 kj the equilibrium constant for this reaction will
a. increase if the temperature is decreased.
b. decrease with increasing temperature.
c. increase with increasing temperature.
d. increase at some pressures and decrease at other pressures.
e. not change if the temperature is increased.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
Consider the following equilibrium: co2(g) + c(graphite) 2co(g); δh = 172.5 kj the equilibrium con...
Questions


Mathematics, 12.04.2021 06:00

Mathematics, 12.04.2021 06:00

English, 12.04.2021 06:00

Mathematics, 12.04.2021 06:00


Mathematics, 12.04.2021 06:00




Mathematics, 12.04.2021 06:00



Mathematics, 12.04.2021 06:00

English, 12.04.2021 06:00

Mathematics, 12.04.2021 06:00

Health, 12.04.2021 06:00

Mathematics, 12.04.2021 06:00


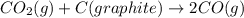
![K_{c} = \frac{[CO_{2}]}{[CO]^{2}}](/tpl/images/0180/4423/2161b.png)


