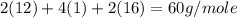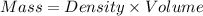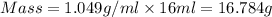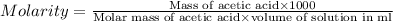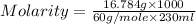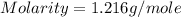Chemistry, 20.08.2019 01:20 anaroles04
Calculate the molarity of a solution of acetic acid made by dissolving 16.00 ml of glacial acetic acid at 25 ∘c in enough water to make 230.0 ml of solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

You know the right answer?
Calculate the molarity of a solution of acetic acid made by dissolving 16.00 ml of glacial acetic ac...
Questions

Mathematics, 12.08.2020 07:01

Biology, 12.08.2020 07:01

Health, 12.08.2020 07:01

Advanced Placement (AP), 12.08.2020 07:01



Physics, 12.08.2020 07:01

English, 12.08.2020 07:01



Chemistry, 12.08.2020 07:01


Mathematics, 12.08.2020 07:01



Chemistry, 12.08.2020 07:01

Arts, 12.08.2020 07:01

Computers and Technology, 12.08.2020 07:01

Mathematics, 12.08.2020 07:01

Mathematics, 12.08.2020 07:01

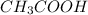 =
=