Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
The heat of formation of fe2o3(s) is –826.0 kj/mol. calculate the heat of the reaction 4fe(s) + 3o2(...
Questions


Mathematics, 21.11.2019 05:31


Mathematics, 21.11.2019 05:31



Mathematics, 21.11.2019 05:31

Social Studies, 21.11.2019 05:31

Mathematics, 21.11.2019 05:31


Biology, 21.11.2019 05:31


Mathematics, 21.11.2019 05:31


Mathematics, 21.11.2019 05:31


Mathematics, 21.11.2019 05:31


History, 21.11.2019 05:31

Geography, 21.11.2019 05:31

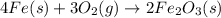
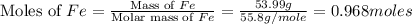
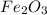
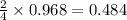 moles of
moles of