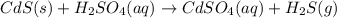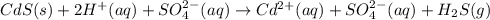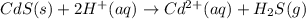Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
Solid cadmium sulfide reacts with an aqueous solution of sulfuric acid . express your answer as a ba...
Questions

English, 03.12.2019 22:31


Mathematics, 03.12.2019 22:31


English, 03.12.2019 22:31





Mathematics, 03.12.2019 22:31

Spanish, 03.12.2019 22:31

Mathematics, 03.12.2019 22:31

English, 03.12.2019 22:31

Biology, 03.12.2019 22:31


History, 03.12.2019 22:31

Mathematics, 03.12.2019 22:31

Mathematics, 03.12.2019 22:31