
Chemistry, 19.08.2019 19:30 2020seogang
Dalton's law of partial pressures states that the total pressure exerted by a mixture of gases is the sum of the pressures exerted independently by each gas in the mixture. true false

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1


Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
Dalton's law of partial pressures states that the total pressure exerted by a mixture of gases is th...
Questions


English, 07.10.2019 18:20




English, 07.10.2019 18:20

History, 07.10.2019 18:20


English, 07.10.2019 18:30

Mathematics, 07.10.2019 18:30

Mathematics, 07.10.2019 18:30


Computers and Technology, 07.10.2019 18:30

Biology, 07.10.2019 18:30


Biology, 07.10.2019 18:30



Mathematics, 07.10.2019 18:30

Physics, 07.10.2019 18:30

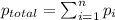
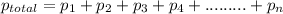
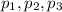 ....... = partial pressure of individual gases present in the mixture
....... = partial pressure of individual gases present in the mixture 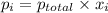
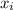 = mole fraction
= mole fraction

