
Chemistry, 19.08.2019 19:30 carolinagarciap8kgmi
Propanoic acid has a boiling point of 141 °c, propanamide has a boiling point of 213 °c, and propanal has a boiling point of 48 °c. rationalize the differences in boiling point between these three compounds.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Propanoic acid has a boiling point of 141 °c, propanamide has a boiling point of 213 °c, and propana...
Questions

Mathematics, 21.05.2021 01:50


Mathematics, 21.05.2021 01:50


World Languages, 21.05.2021 01:50

Mathematics, 21.05.2021 01:50

Mathematics, 21.05.2021 01:50

Mathematics, 21.05.2021 01:50



Mathematics, 21.05.2021 01:50

Mathematics, 21.05.2021 01:50

Mathematics, 21.05.2021 01:50




Mathematics, 21.05.2021 01:50



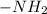 , the hydrogen atoms which are present are positive in nature. Due to this they are able to form hydrogen bonds with the neighboring oxygen atom.
, the hydrogen atoms which are present are positive in nature. Due to this they are able to form hydrogen bonds with the neighboring oxygen atom. 

