
Chloral hydrate (c2h3cl3o2) is a drug formerly used as a sedative and hypnotic.
a. calculate the molar mass of chloral hydrate. b. what amount (moles) of c2h3cl3o2 molecules are in 500.0 g chloral hydrate? c. what is the mass in grams of 2.0 x 10-2 mol chloral hydrate? d. what number of chlorine atoms are in 5.0 g chloral hydrate? e. what mass of chloral hydrate would contain 1.0 g cl? f. what is the mass of exactly 500 molecules of chloral hydrate?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
Chloral hydrate (c2h3cl3o2) is a drug formerly used as a sedative and hypnotic.
a. calculate...
a. calculate...
Questions


English, 23.09.2019 09:30


Biology, 23.09.2019 09:30



Chemistry, 23.09.2019 09:30

Mathematics, 23.09.2019 09:30




Mathematics, 23.09.2019 09:30

Mathematics, 23.09.2019 09:30

Mathematics, 23.09.2019 09:30

Mathematics, 23.09.2019 09:30

Physics, 23.09.2019 09:30


Biology, 23.09.2019 09:30


Mathematics, 23.09.2019 09:30

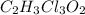 is, 165.5 g/mole
is, 165.5 g/mole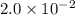 mole chloral hydrate is, 3.31 g
mole chloral hydrate is, 3.31 g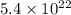

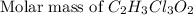 =2(12g/mole)+3(1g/mole)+3(35.5g/mole)+2(16g/mole)=165.5g/mole[/tex]
=2(12g/mole)+3(1g/mole)+3(35.5g/mole)+2(16g/mole)=165.5g/mole[/tex]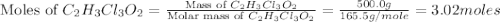
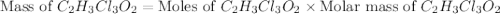
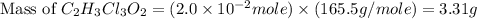
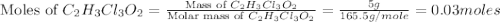
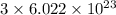 chlorine atoms
chlorine atoms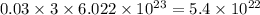 chlorine atoms
chlorine atoms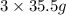 of chlorine present in 165.5 g of
of chlorine present in 165.5 g of 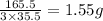 of
of 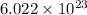 molecules of chloral hydrate has 165.5 g mass of chloral hydrate
molecules of chloral hydrate has 165.5 g mass of chloral hydrate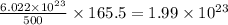 mass of chloral hydrate
mass of chloral hydrate

