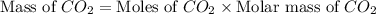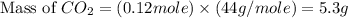Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 1.74 g of butane is mixed with 11. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1
You know the right answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions


Mathematics, 21.12.2019 06:31

English, 21.12.2019 06:31






Mathematics, 21.12.2019 07:31

Mathematics, 21.12.2019 07:31






English, 21.12.2019 07:31


English, 21.12.2019 07:31

Advanced Placement (AP), 21.12.2019 07:31

 produced will be, 5.3 grams.
produced will be, 5.3 grams.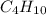 = 1.74 g
= 1.74 g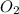 = 11 g
= 11 g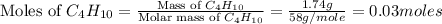
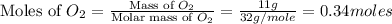
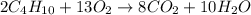
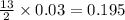 moles of
moles of 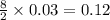 moles of
moles of