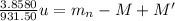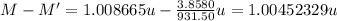Two isotopes of a certain element have binding energies that differ by 3.8580 mev. the isotope with the larger binding energy contains one more neutron than the other isotope. find the difference in atomic mass between the two isotopes, by taking the energy equivalent of 1 u to be 931.50 mev. express your answer in atomic mass units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Two isotopes of a certain element have binding energies that differ by 3.8580 mev. the isotope with...
Questions

Mathematics, 17.01.2021 06:20







Mathematics, 17.01.2021 06:20




Mathematics, 17.01.2021 06:20

English, 17.01.2021 06:20


Mathematics, 17.01.2021 06:20



Mathematics, 17.01.2021 06:20


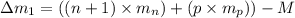
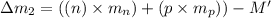
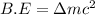 (general binding energy expression)
(general binding energy expression)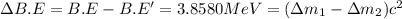 ..(1)
..(1)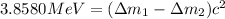
![=([((n+1)\times m_n)+(p\times m_p))-M]-[((n)\times m_n)+(p\times m_p))-M'])c^2](/tpl/images/0175/4824/623d3.png)
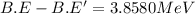
![3.8580 MeV=[1\times m_n-M+M']c^2](/tpl/images/0175/4824/8fa3b.png)