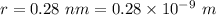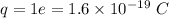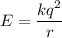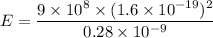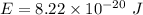Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Why is it illegal to manufacture fireworks without a license
Answers: 1

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1


Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2
You know the right answer?
Estimate the binding energy of a kcl molecule by calculating the electrostatic potential energy when...
Questions

English, 16.02.2021 02:50

Mathematics, 16.02.2021 02:50





Mathematics, 16.02.2021 02:50


History, 16.02.2021 02:50

History, 16.02.2021 02:50

Mathematics, 16.02.2021 02:50

Mathematics, 16.02.2021 02:50


Computers and Technology, 16.02.2021 02:50




Mathematics, 16.02.2021 02:50