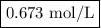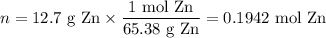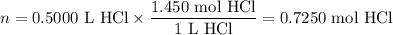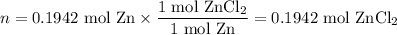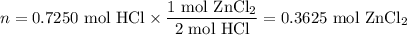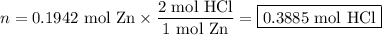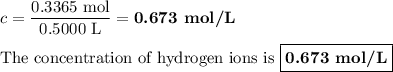Chemistry, 16.08.2019 08:20 JAYDENJONES0111
Zinc dissolves in hydrochloric acid to yield hydrogen gas: zn(s) + 2hcl(aq) --> zncl2(aq) + h2(g) when a 12.7 g chunk of zinc dissolves in 5.00 x 102 ml of 1.450 m hcl, what is the concentration of hydrogen ions remaining in the final solution? 0 m0.388 m0.674 m0.776 m1.06 m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3


Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
Zinc dissolves in hydrochloric acid to yield hydrogen gas: zn(s) + 2hcl(aq) --> zncl2(aq) + h2(...
Questions

Mathematics, 09.02.2022 22:10


Chemistry, 09.02.2022 22:10


Mathematics, 09.02.2022 22:10

English, 09.02.2022 22:10

History, 09.02.2022 22:10

Mathematics, 09.02.2022 22:10

Biology, 09.02.2022 22:10





Geography, 09.02.2022 22:10



Mathematics, 09.02.2022 22:20