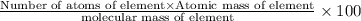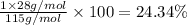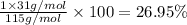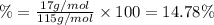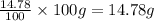Chemistry, 14.08.2019 08:10 anoyinpokep3c3sg
Ammonium dihydrogen phosphate, formed from the reaction of phosphoric acid with ammonia, is used as a crop fertilizer as well as a component of some fire extinguishers, (a) what are the mass percentages of n and p in the compound? (b) how much ammonia is incorporated into 100. g of compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 23.06.2019 22:00
The following compounds, listed with their boiling points, are liquid at –10ºc: butane, –0.5ºc; ethanol, 78.3ºc; toluene, 110.6ºc. at –10ºc, which of these liquids would you expect to have the highest vapor pressure? which the lowest? explain. (4 points)
Answers: 1

Chemistry, 23.06.2019 23:50
55. streptomycin is a derivative of aa. peptides b. carbohydratesd. terenes c.purines
Answers: 1

Chemistry, 24.06.2019 00:00
Which statements correctly match a chemical name with its formula? use the list of polyatomic ions and the periodic table to you answer. the chemical formula for ammonium carbonate is nh4hco3. the chemical formula for ammonium hypochlorite is nh4clo. the chemical formula for ammonium nitrate is nh4no3. the chemical formula for ammonium phosphate is nh4(po4)3. the chemical formula for ammonium sulfate is (nh4)2so3.
Answers: 3
You know the right answer?
Ammonium dihydrogen phosphate, formed from the reaction of phosphoric acid with ammonia, is used as...
Questions


Geography, 27.01.2020 18:31





Mathematics, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31


Mathematics, 27.01.2020 18:31

History, 27.01.2020 18:31


Mathematics, 27.01.2020 18:31




History, 27.01.2020 18:31


Business, 27.01.2020 18:31

 .
.