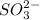Chemistry, 13.08.2019 05:30 naysia2006
Deduce the formulas of the following ionic compounds: (a) iron(lll) sulfide, (b) mercury(ll) nitrate, and (c) potassium sulfite.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1
You know the right answer?
Deduce the formulas of the following ionic compounds: (a) iron(lll) sulfide, (b) mercury(ll) nitrat...
Questions



Mathematics, 02.02.2021 14:00


History, 02.02.2021 14:00

English, 02.02.2021 14:00


Mathematics, 02.02.2021 14:00

Mathematics, 02.02.2021 14:00

Physics, 02.02.2021 14:00



Chemistry, 02.02.2021 14:00


Mathematics, 02.02.2021 14:00




Mathematics, 02.02.2021 14:00

History, 02.02.2021 14:00

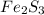
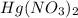
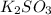
![[Ar]3d^64s^2](/tpl/images/0174/8186/7fd67.png) .
.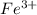 ion, this element will loose 3 electrons.
ion, this element will loose 3 electrons.![[Ne]3s^23p^4](/tpl/images/0174/8186/9a0fd.png) .
.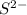 ion, this element will gain 2 electrons.
ion, this element will gain 2 electrons. ![[Xe]4f^{14}5d^{10}6s^2](/tpl/images/0174/8186/13e4d.png) .
.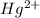 ion, this element will loose 2 electrons.
ion, this element will loose 2 electrons.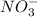
![[Ar]4s^1](/tpl/images/0174/8186/85db5.png) .
.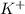 ion, this element will loose 1 electron.
ion, this element will loose 1 electron.