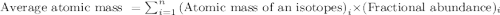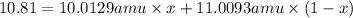Chemistry, 13.08.2019 05:30 luclaymom805
Boron has two naturally occurring isotopes, 10b and 11b, which have masses 10.0129 and 11.0093 amu, respectively. given the average atomic mass of boron (10.81 amu), determine the percent abundance of each isotope. (a) 50%10b, 50%11b (b) 20%10b, 80%11b (c) 98%10b, 2% 11b (d) 93%10b, 7%11b (e) 22%10b, 78%11b

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1
You know the right answer?
Boron has two naturally occurring isotopes, 10b and 11b, which have masses 10.0129 and 11.0093 amu,...
Questions

Biology, 02.10.2019 02:40

Biology, 02.10.2019 02:40

English, 02.10.2019 02:40


Mathematics, 02.10.2019 02:40


World Languages, 02.10.2019 02:40

Mathematics, 02.10.2019 02:40



Mathematics, 02.10.2019 02:40

Business, 02.10.2019 02:40





Mathematics, 02.10.2019 02:40


Social Studies, 02.10.2019 02:40

English, 02.10.2019 02:40