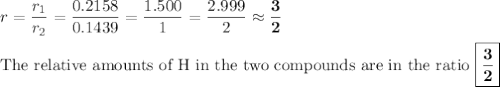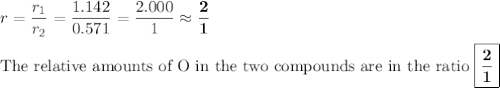Chemistry, 13.08.2019 05:20 quanharris2k19
In each case, calculate the appropriate ratio to show that the information given is consistent with the law of multiple proportions. (a) both ammonia (nh3) and hydrazine (n2h4) are composed of nitrogen and hydrogen. ammonia contains 0.2158 g hydrogen for every gram of nitrogen. hydrazine contains 0.1439 g hydrogen for every gram of nitrogen. (b) two of the compounds that consist of nitrogen and oxygen are nitric oxide, also known as nitrogen monoxide (no) and nitrous oxide (n20), which is also known as dinitrogen monoxide. nitric oxide contains 1.142 g oxygen for every gram of nitrogen. nitrous oxide contains 0.571 g oxygen for every gram of nitrogen.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 14:20
Identificaa 5 características que comparten las guacamayas y dos caracteristicas que las hagan diferentes
Answers: 1
You know the right answer?
In each case, calculate the appropriate ratio to show that the information given is consistent with...
Questions


History, 05.05.2020 13:42

Chemistry, 05.05.2020 13:42

Chemistry, 05.05.2020 13:42



Chemistry, 05.05.2020 13:42

Mathematics, 05.05.2020 13:42

Mathematics, 05.05.2020 13:42

English, 05.05.2020 13:42




English, 05.05.2020 13:42

Computers and Technology, 05.05.2020 13:42



Mathematics, 05.05.2020 13:42