

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2

Chemistry, 23.06.2019 13:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 13:30
Use the periodic table to classify each of the elements below. cadmium (cd): vanadium (v): xenon (xe): iodine (i): potassium (k): strontium (sr):
Answers: 3
You know the right answer?
Write empirical formulas for the following molecules: (a) caffeine (c8h10n4o2), a stimulant found i...
Questions

Social Studies, 04.01.2021 22:00




English, 04.01.2021 22:00

Mathematics, 04.01.2021 22:00


Business, 04.01.2021 22:00

History, 04.01.2021 22:00

Mathematics, 04.01.2021 22:00



Mathematics, 04.01.2021 22:00


Mathematics, 04.01.2021 22:00

Mathematics, 04.01.2021 22:00

Mathematics, 04.01.2021 22:00

Health, 04.01.2021 22:00

Mathematics, 04.01.2021 22:00

History, 04.01.2021 22:00

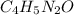
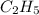
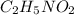
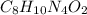 has empirical formula of
has empirical formula of 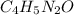
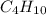 has an empirical formula of
has an empirical formula of 

