
Chemistry, 13.08.2019 04:30 emmaguentherp3hjd3
The oxidation of ammonia produces nitrogen and water via the following reaction: 4nh3(g) + 3o2(g) → 2n2(g) + 6h2o(l) suppose the rate of formation of h2o(l) is 3.0 mol/(l ∙ s). which of the following statements is true? a. the rate of consumption of nh3 is 0.50 mol/(l ∙ s). b. the rate of consumption of nh3 is 2.0 mol/(l ∙ s). c. the rate of formation of n2 is 2.0 mol/(l ∙ s). d. the rate of formation of n2 is 1.3 mol/(l ∙ s). e. the rate of consumption of o2 is 2.0 mol/(l ∙ s).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1


Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
The oxidation of ammonia produces nitrogen and water via the following reaction: 4nh3(g) + 3o2(g) →...
Questions

Advanced Placement (AP), 18.12.2019 12:31

Mathematics, 18.12.2019 12:31




History, 18.12.2019 12:31



Mathematics, 18.12.2019 12:31

Social Studies, 18.12.2019 12:31

Mathematics, 18.12.2019 12:31

Mathematics, 18.12.2019 12:31

English, 18.12.2019 12:31

Chemistry, 18.12.2019 12:31


Biology, 18.12.2019 12:31

Health, 18.12.2019 12:31

Computers and Technology, 18.12.2019 12:31

Chemistry, 18.12.2019 12:31

Physics, 18.12.2019 12:31

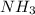 is 2.0 mol/L.s
is 2.0 mol/L.s![-\frac{1}{4}\frac{\Delta [NH_{3}]}{\Delta t}=-\frac{1}{3}\frac{\Delta [O_{2}]}{\Delta t}=\frac{1}{2}\frac{\Delta [N_{2}]}{\Delta t}=\frac{1}{6}\frac{\Delta [H_{2}O]}{\Delta t}](/tpl/images/0174/7635/4514d.png)
![-\frac{\Delta [NH_{3}]}{\Delta t}](/tpl/images/0174/7635/80478.png) represents rate of consumption of
represents rate of consumption of ![-\frac{\Delta [O_{2}]}{\Delta t}](/tpl/images/0174/7635/8d0a6.png) represents rate of consumption of
represents rate of consumption of 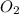 ,
, ![\frac{\Delta [N_{2}]}{\Delta t}](/tpl/images/0174/7635/cd482.png) represents rate of formation of
represents rate of formation of 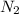 and
and ![\frac{\Delta [H_{2}O]}{\Delta t}](/tpl/images/0174/7635/668d2.png) represents rate of formation of
represents rate of formation of 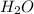 .
.![-\frac{1}{4}\frac{\Delta [NH_{3}]}{\Delta t}=\frac{1}{6}\frac{\Delta [H_{2}O]}{\Delta t}](/tpl/images/0174/7635/898d9.png)
![\frac{\Delta [H_{2}O]}{\Delta t}=3.0 mol/(L.s))](/tpl/images/0174/7635/830f4.png)
![-\frac{\Delta [NH_{3}]}{\Delta t}=\frac{4}{6}\frac{\Delta [H_{2}O]}{\Delta t}](/tpl/images/0174/7635/d2499.png)
![-\frac{\Delta [NH_{3}]}{\Delta t}=\frac{4}{6}\times 3.0 mol/(L.s)=2.0 mol/(L.s)](/tpl/images/0174/7635/32306.png)


