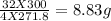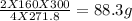Chemistry, 13.08.2019 03:30 tddreviews
4fecr2o7 + 8 k2co3 + o2 2 fe2o3 + 8 k2cro4 + 8 co2 how many grams of iron (ii) dichromate are required to produce 44.0 grams of carbon dioxide? how many grams of oxygen gas are required to produce 100.0 grams of ferric oxide? if 300.0 grams of iron (ii) dichromate react, how many grams of oxygen gas will be consumed? how many grams of iron (iii) oxide will be produced from 300.0 grams of ferrous dichromate?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
4fecr2o7 + 8 k2co3 + o2 2 fe2o3 + 8 k2cro4 + 8 co2 how many grams of iron (ii) dichromate are requi...
Questions



Law, 12.10.2020 23:01




History, 12.10.2020 23:01



Medicine, 12.10.2020 23:01


Mathematics, 12.10.2020 23:01


Biology, 12.10.2020 23:01


History, 12.10.2020 23:01

Mathematics, 12.10.2020 23:01


Spanish, 12.10.2020 23:01

Mathematics, 12.10.2020 23:01