
Apossible mechanism for the gas phase reaction of no and h2 is as follows: step 1 2no n2o2 step 2 n2o2 + h2 n2o + h2o step 3 n2o + h2 n2 + h2o which of the following statements concerning this mechanism is not directly supported by the information provided? a. n2o2 is an intermediate. b. all steps are bimolecular reactions. c. the rate expression for step 1 is rate = k[no]2. d. step 1 is the rate determining step. e. there is no catalyst in this reaction. 1 points question 9

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
Apossible mechanism for the gas phase reaction of no and h2 is as follows: step 1 2no n2o2 step 2 n...
Questions



Mathematics, 21.01.2022 14:00


Business, 21.01.2022 14:00

English, 21.01.2022 14:00

Biology, 21.01.2022 14:00


Social Studies, 21.01.2022 14:00



Computers and Technology, 21.01.2022 14:00

Mathematics, 21.01.2022 14:00


English, 21.01.2022 14:00




History, 21.01.2022 14:00

Mathematics, 21.01.2022 14:00

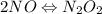 (fast)
(fast)![[NO]^{2}](/tpl/images/0174/7336/f0d3d.png)
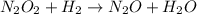
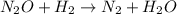 (fast)
(fast)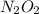 is intermediate in nature.
is intermediate in nature.

