
Chemistry, 13.08.2019 03:30 armonilucky11
Balance each of the following redox reactions occurring in basic solution. mno−4(aq)+br−(aq)→mno2(s)+bro−3(aq) express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
Balance each of the following redox reactions occurring in basic solution. mno−4(aq)+br−(aq)→mno2(s)...
Questions


Mathematics, 18.03.2021 02:30


Physics, 18.03.2021 02:30




Mathematics, 18.03.2021 02:30


Chemistry, 18.03.2021 02:30

History, 18.03.2021 02:30







Chemistry, 18.03.2021 02:30


Mathematics, 18.03.2021 02:30

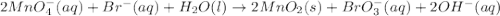
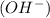 at that side where the less number of hydrogen are present.Now balance the charge.
at that side where the less number of hydrogen are present.Now balance the charge.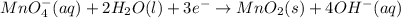 ......(1)
......(1)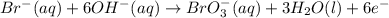 .......(2)
.......(2)

