
Chemistry, 13.08.2019 03:30 nolandh7940
Write the empirical formulas of the following compounds: (a) c2n2, (b) c6h6, (c) c9h2o, (d) p4o10, (e) b2h6.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
Write the empirical formulas of the following compounds: (a) c2n2, (b) c6h6, (c) c9h2o, (d) p4o10,...
Questions

Business, 23.08.2019 04:30




Geography, 23.08.2019 04:40

Mathematics, 23.08.2019 04:40

Mathematics, 23.08.2019 04:40


Mathematics, 23.08.2019 04:40

Mathematics, 23.08.2019 04:40


Mathematics, 23.08.2019 04:40

Mathematics, 23.08.2019 04:40

Biology, 23.08.2019 04:40






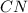
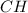
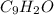
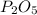
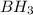
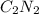 has an empirical formula of
has an empirical formula of 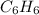 has an empirical formula of
has an empirical formula of 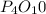 has an empirical formula of
has an empirical formula of 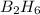 has an empirical formula of
has an empirical formula of 

