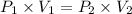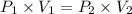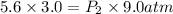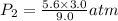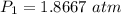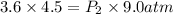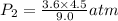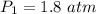Chemistry, 13.08.2019 03:10 OliviaParis8837
Asample of he gas (3.0 l) at 5.6 atm and 25°c was combined with 4.5 l of ne gas at 3.6 atm and 25°c at constant temperature in a 9.0 l flask. the total pressure in the flask was atm. assume the initial pressure in the flask was 0.00 atm.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
Asample of he gas (3.0 l) at 5.6 atm and 25°c was combined with 4.5 l of ne gas at 3.6 atm and 25°c...
Questions


History, 25.05.2020 23:59

Biology, 25.05.2020 23:59

Mathematics, 25.05.2020 23:59

History, 25.05.2020 23:59

Biology, 25.05.2020 23:59

Mathematics, 25.05.2020 23:59

Mathematics, 25.05.2020 23:59


English, 25.05.2020 23:59

Mathematics, 25.05.2020 23:59


Chemistry, 26.05.2020 00:00

Mathematics, 26.05.2020 00:00


Mathematics, 26.05.2020 00:00

English, 26.05.2020 00:00

Chemistry, 26.05.2020 00:00