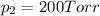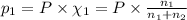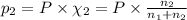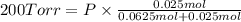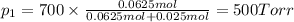Chemistry, 13.08.2019 02:30 lailahussain99
Agas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40.0). if the partial pressure of argon is 200. torr, what is the pressure of methane, in torr? hint: what is the mole fraction of each gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1


Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2
You know the right answer?
Agas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40...
Questions

Mathematics, 12.08.2020 08:01


English, 12.08.2020 08:01







Mathematics, 12.08.2020 08:01


Mathematics, 12.08.2020 08:01






Physics, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01

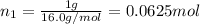
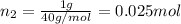
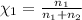
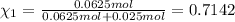
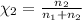
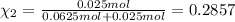
 .
.