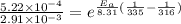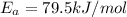Chemistry, 13.08.2019 02:20 dramaqueenactr2040
The rate constants for the first-order decomposition of a compound are 5.22× 10–4 s–1 at 43°c and 2.91 × 10–3 s–1 at 62°c. what is the value of the activation energy for this reaction? (r = 8.31 j/(mol · k)) a. 79.5 kj/mol b. 34.5 kj/mol c. 0.751 kj/mol d. 0.87104 kj/mol e. 2 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
The rate constants for the first-order decomposition of a compound are 5.22× 10–4 s–1 at 43°c and 2....
Questions

Mathematics, 10.10.2019 09:50

Biology, 10.10.2019 09:50

Geography, 10.10.2019 09:50


History, 10.10.2019 09:50

Spanish, 10.10.2019 09:50




Biology, 10.10.2019 09:50

Mathematics, 10.10.2019 09:50

Chemistry, 10.10.2019 09:50

Mathematics, 10.10.2019 09:50

Computers and Technology, 10.10.2019 09:50

Mathematics, 10.10.2019 09:50




Mathematics, 10.10.2019 09:50

History, 10.10.2019 09:50

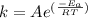
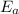 is the activation energy and T is temperature in kelvin
is the activation energy and T is temperature in kelvin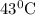 ,
, 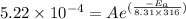 ............(1)
............(1)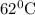 ,
, 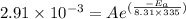 ............(2)
............(2)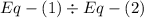 gives-
gives-