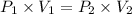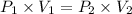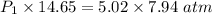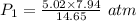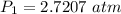Chemistry, 13.08.2019 01:30 hannahkharel2
The volume of a sample of hydrogen gas was decreased from 14.65 l to 7.94 l at constant temperature. if the final pressure exerted by the hydrogen gas sample was 5.02 atm, what pressure did the hydrogen gas exert before its volume was decreased?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1


Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
You know the right answer?
The volume of a sample of hydrogen gas was decreased from 14.65 l to 7.94 l at constant temperature....
Questions

Mathematics, 30.06.2019 04:00


Mathematics, 30.06.2019 04:00

Social Studies, 30.06.2019 04:00






Mathematics, 30.06.2019 04:00




Computers and Technology, 30.06.2019 04:00


Mathematics, 30.06.2019 04:00




English, 30.06.2019 04:00