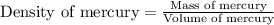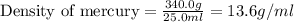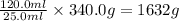Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3



Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Given that 25.0 ml of mercury has a mass of 340.0 g, calculate (a) the density of mercury and (b) th...
Questions


English, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31


Mathematics, 28.01.2020 05:31


History, 28.01.2020 05:31



English, 28.01.2020 05:31


Health, 28.01.2020 05:31


Mathematics, 28.01.2020 05:31

English, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31


Health, 28.01.2020 05:31


Mathematics, 28.01.2020 05:31