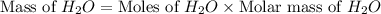Chemistry, 12.08.2019 23:30 nmartin5185
If 50.0 dm3 of methane, ch4, react with 10.0 dm3 of air, calculate the grams of water produced.
ch4 (g) + 2 o2 (g) --> co2 (g) + 2 h2o (l)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3


Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
If 50.0 dm3 of methane, ch4, react with 10.0 dm3 of air, calculate the grams of water produced.
Questions


Mathematics, 18.03.2021 02:50



Mathematics, 18.03.2021 02:50

History, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50





Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Social Studies, 18.03.2021 02:50

English, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50

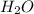 produced will be, 8.1 grams.
produced will be, 8.1 grams.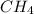 =
= 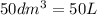
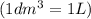
 =
= 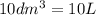
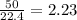 mole of
mole of 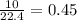 mole of
mole of 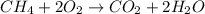
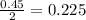 moles of
moles of