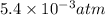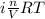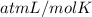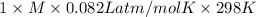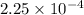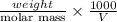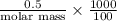Chemistry, 12.08.2019 22:20 cravingnafi202
Asolution of 0.5 g of an unknown nonvolatile, nonelectrolyte solute is added to 100 ml of water and then placed across a semipermeable membrane from a volume of pure water. when the system reaches equilibrium, the solution compartment is elevated 5.6 cm above the solvent compartment. assuming that the density of the solution is 1.0 g / ml, calculate the molecular mass of the unknown.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
Asolution of 0.5 g of an unknown nonvolatile, nonelectrolyte solute is added to 100 ml of water and...
Questions

Biology, 09.11.2020 18:20


Mathematics, 09.11.2020 18:20

Mathematics, 09.11.2020 18:20

Mathematics, 09.11.2020 18:20


Advanced Placement (AP), 09.11.2020 18:20

Arts, 09.11.2020 18:20



Mathematics, 09.11.2020 18:20

History, 09.11.2020 18:20

English, 09.11.2020 18:20

History, 09.11.2020 18:20


Mathematics, 09.11.2020 18:20


Mathematics, 09.11.2020 18:20


Social Studies, 09.11.2020 18:20

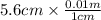 = 0.056, density = 1.0 g/ml, g = 9.8 m/s
= 0.056, density = 1.0 g/ml, g = 9.8 m/s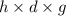
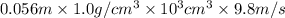
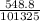 atm
atm