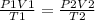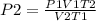Answers: 3


Another question on Chemistry




Chemistry, 23.06.2019 19:00
Covalent bonds have a higher melting points than ionic bonds. true or false
Answers: 1
You know the right answer?
Consider 4.60 l of a gas at 365 mmhg and 20. ∘c . if the container is compressed to 2.60 l and the t...
Questions


Mathematics, 17.11.2020 22:40

Social Studies, 17.11.2020 22:40

Mathematics, 17.11.2020 22:40


Social Studies, 17.11.2020 22:40

English, 17.11.2020 22:40

Mathematics, 17.11.2020 22:40

History, 17.11.2020 22:40

Mathematics, 17.11.2020 22:40

History, 17.11.2020 22:40

Mathematics, 17.11.2020 22:40

Mathematics, 17.11.2020 22:40

Mathematics, 17.11.2020 22:40

Mathematics, 17.11.2020 22:40

Mathematics, 17.11.2020 22:40

Mathematics, 17.11.2020 22:40

Mathematics, 17.11.2020 22:40