
Chemistry, 12.08.2019 21:30 rhyanebean6443
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts with oxygen gas, producing carbon dioxide gas and water vapor. calculate the moles of carbon dioxide produced by the reaction of 1.5 mol of oxygen. be sure your answer has a unit symbol, if necessary, and round it to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts...
Questions

Mathematics, 24.02.2021 23:20

Mathematics, 24.02.2021 23:20

Mathematics, 24.02.2021 23:20

Physics, 24.02.2021 23:20



Mathematics, 24.02.2021 23:20



Mathematics, 24.02.2021 23:20


Mathematics, 24.02.2021 23:20


History, 24.02.2021 23:20

Arts, 24.02.2021 23:20

Mathematics, 24.02.2021 23:20




Biology, 24.02.2021 23:20

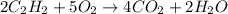
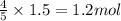 of carbon dioxide.
of carbon dioxide.

