
Chemistry, 12.08.2019 21:20 edfrank6278
In your lab you are studying aspirin, and its acid/base properties. you find that a 1.00 l of a 0.500 m solution of aspirin has a ph of 1.86. you are interested in learning about the % dissociation in a buffered solution of aspirin, so you make a new 1.00 l solution containing 0.500 moles of aspirin and 0.25 moles of the sodium salt of aspirin. what will the % dissociation be in this new buffered solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 24.06.2019 00:00
Suppose that you stir a green powder into a clear liquid. the liquid turns green. you leave the room and come back later to observe that the top of the liquid is clear again. a green substance has settled on the bottom of the container. was the green liquid that you made a compound or a mixture? explain your reasoning.
Answers: 1
You know the right answer?
In your lab you are studying aspirin, and its acid/base properties. you find that a 1.00 l of a 0.50...
Questions




















![-log[H^{+}]](/tpl/images/0174/4805/1d5a1.png)
![[H^{+}] = 10^{-pH}](/tpl/images/0174/4805/241df.png)
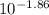
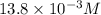
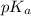 value will be calculated as follows.
value will be calculated as follows.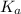 =
= ![\frac{[H^{+}]^{2}}{[Aspirin]}](/tpl/images/0174/4805/0efaa.png)
![\frac{[13.8 \times 10^{-3}]^{2}}{0.50}](/tpl/images/0174/4805/d6d3a.png)
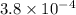
![pK_{a} = -log [K_{a}]](/tpl/images/0174/4805/95c79.png)
![-log [3.8 \times 10^{-4}]](/tpl/images/0174/4805/46d55.png)
![pK_{a} + log \frac{[CH_{3}COO^{-}]}{[Aspirin]}](/tpl/images/0174/4805/cc4ea.png)
![log \frac{[0.25]}{[0.5]}](/tpl/images/0174/4805/52faa.png)
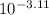
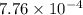 M
M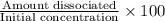
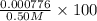
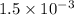 × 100
× 100

