
Chemistry, 12.08.2019 20:30 ferguag15stu
The following data were obtained in a kinetics study of the hypothetical reaction a + b + c → products. [a]0 (m) [b]0 (m) [c]0 (m) initial rate (10–3 m/s) 0.4 0.4 0.2 160 0.2 0.4 0.4 80 0.6 0.1 0.2 15 0.2 0.1 0.2 5 0.2 0.2 0.4 20 using the initial-rate method, what is the order of the reaction with respect to c? a. zero-order b. first-order c. third-order d. second-order e. impossible to tell from the data given

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
The following data were obtained in a kinetics study of the hypothetical reaction a + b + c → produc...
Questions


Mathematics, 29.08.2019 04:10




Mathematics, 29.08.2019 04:10

Mathematics, 29.08.2019 04:10

History, 29.08.2019 04:10

Mathematics, 29.08.2019 04:10



History, 29.08.2019 04:10

Mathematics, 29.08.2019 04:10


Mathematics, 29.08.2019 04:10

Mathematics, 29.08.2019 04:10

History, 29.08.2019 04:10

Social Studies, 29.08.2019 04:10

SAT, 29.08.2019 04:10

Mathematics, 29.08.2019 04:10

![Rate = k[A]^{x}[B]^{y}[C]^{z}](/tpl/images/0174/4444/51b37.png)
![\frac{Rate3}{Rate4}= [\frac{[A(3)]}{[A(4)]}]^{x}](/tpl/images/0174/4444/34c33.png)
![\frac{15}{5}= [\frac{[0.6]}{[0.2]}]^{x}](/tpl/images/0174/4444/c782c.png)

![\frac{Rate2}{Rate5}= [\frac{[B(2)]}{[B(5)]}]^{y}](/tpl/images/0174/4444/f0ddb.png)
![\frac{80}{20}= [\frac{[0.4]}{[0.2]}]^{y}](/tpl/images/0174/4444/8ed1b.png)
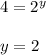
![\frac{Rate1}{Rate2}= [\frac{[A(1)]}{[A(2)]}]^{x}[\frac{[B(1)]}{[B(2)]}]^{y}[\frac{[C(1)]}{[C(2)]}]^{z}](/tpl/images/0174/4444/66487.png)
![\frac{160}{80}= [\frac{[0.4]}{[0.2]}]^{1}[\frac{[0.4]}{[0.4]}]^{2}[\frac{[0.2]}{[0.4]}]^{z}](/tpl/images/0174/4444/7ba82.png)



