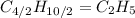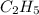Chemistry, 12.08.2019 19:30 lizzyhearts
Determine the empirical formulas for the following compounds:
(a) acetic acid, c24o2
(b) citric acid, c6h8o7
(c) hydrazine, n2h4
(d) nicotine, c10h14n2
(e) butane, c4h10

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

You know the right answer?
Determine the empirical formulas for the following compounds:
(a) acetic acid, c24o2
(b...
(a) acetic acid, c24o2
(b...
Questions

Mathematics, 17.11.2020 21:00





History, 17.11.2020 21:00

Mathematics, 17.11.2020 21:00

Mathematics, 17.11.2020 21:00

Computers and Technology, 17.11.2020 21:00

Physics, 17.11.2020 21:00


English, 17.11.2020 21:00


Mathematics, 17.11.2020 21:00


History, 17.11.2020 21:00



Mathematics, 17.11.2020 21:00

Mathematics, 17.11.2020 21:00

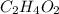 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.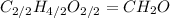
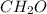
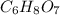 . To write the empirical formula, we divide each subscript by 1.
. To write the empirical formula, we divide each subscript by 1.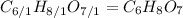
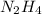 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.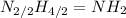
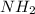
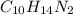 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.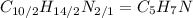
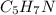
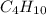 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.