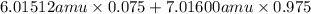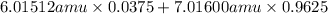Chemistry, 12.08.2019 19:20 kaywendel2008
Variations in average atomic mass may be observed for elements obtained from different sources. lithium provides an example of this. the isotopic composition of lithium from naturally occurring minerals is 7.5% 6li and 92.5% 7li, which have masses of 6.01512 amu and 7.01600 amu, respectively. a commercial source of lithium, recycled from a military source, was 3.75% 6li (and the rest 7li). calculate the average atomic mass values for each of these two sources.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Variations in average atomic mass may be observed for elements obtained from different sources. lith...
Questions

Mathematics, 29.01.2020 18:46



Mathematics, 29.01.2020 18:46

Chemistry, 29.01.2020 18:46



Mathematics, 29.01.2020 18:46

Mathematics, 29.01.2020 18:46

Biology, 29.01.2020 18:46

History, 29.01.2020 18:46

Biology, 29.01.2020 18:46

Mathematics, 29.01.2020 18:46




Social Studies, 29.01.2020 18:46


History, 29.01.2020 18:46