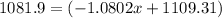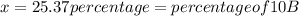The average atomic masses of some elements may vary, depending upon souces of their ores. naturally occurring boron consist of two isotopes with accurately known masses (10 b, 10.0129 amu and 11b, 11.0931 amu). the actual atomic mass of boron can vary grom 10.807 to 10819, depending on whether the mineral source is from turkey or the united states. calculate the percent abundances leading to the two values of the average atomic masses of boron from these two countries.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2


Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
The average atomic masses of some elements may vary, depending upon souces of their ores. naturally...
Questions

Mathematics, 12.10.2020 22:01

Mathematics, 12.10.2020 22:01




Mathematics, 12.10.2020 22:01



Physics, 12.10.2020 22:01

Mathematics, 12.10.2020 22:01



Physics, 12.10.2020 22:01



Arts, 12.10.2020 22:01




Social Studies, 12.10.2020 22:01

![\frac{[percentageofisotope(1)Xatomicmassofisotope(1)]+[percentageofisotope(2)Xatomicmassofisotope(2)}{100}](/tpl/images/0174/3972/b6fa0.png)
![10.807=\frac{[x(10.0129)]+[(100-x)11.0931]}{100}=\frac{10.0129x+1109.31-11.0931x}{100}](/tpl/images/0174/3972/4e3aa.png)
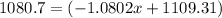
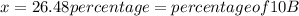
![10.819=\frac{[x(10.0129)]+[(100-x)11.0931]}{100}=\frac{10.0129x+1109.31-11.0931x}{100}](/tpl/images/0174/3972/7b5b5.png)