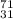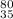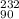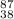Write the symbol for each of the following ions:
(a) the ion with a 3+ charge, 28 electrons,...

Chemistry, 12.08.2019 19:10 jackiecroce1
Write the symbol for each of the following ions:
(a) the ion with a 3+ charge, 28 electrons, and a mass number of 71
(b) the ion with 36 electrons, 35 protons, and 45 neutrons
(c) the ion with 86 electrons,142 neutrons, and a 4+ charge
(d) the ion with a 2+ charge, atomic number 38, and mass number 87

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
Questions




Mathematics, 21.09.2020 05:01

Chemistry, 21.09.2020 05:01







Mathematics, 21.09.2020 05:01

Mathematics, 21.09.2020 05:01



Mathematics, 21.09.2020 05:01

History, 21.09.2020 05:01

Mathematics, 21.09.2020 05:01


Chemistry, 21.09.2020 05:01