
Chemistry, 12.08.2019 18:30 briarkaltvedt
Calculate δ h° for the reaction c 4h 4( g) + 2h 2( g) → c 4h 8( g), using the following data: δ h° combustion for c 4h 4( g) = –2341 kj/mol δ h° combustion for h 2( g) = –286 kj/mol δ h° combustion for c 4h 8( g) = –2755 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1


Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
Calculate δ h° for the reaction c 4h 4( g) + 2h 2( g) → c 4h 8( g), using the following data: δ h°...
Questions


History, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01

History, 11.10.2020 02:01


Mathematics, 11.10.2020 02:01

Chemistry, 11.10.2020 02:01



Mathematics, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01



Mathematics, 11.10.2020 02:01

Business, 11.10.2020 02:01


Mathematics, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01

Arts, 11.10.2020 02:01

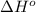
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_{(product)}]-\sum [n\times \Delta H^o_{(reactant)}]](/tpl/images/0174/3643/6872e.png)
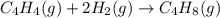
![\Delta H^o_{rxn}=[(1\times \Delta H^o_{(C_4H_8(g))})]-[(1\times \Delta H^o_{(C_4H_4(g))})+(2\times \Delta H^o_{(H_2(g))})]](/tpl/images/0174/3643/605f9.png)
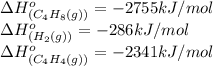
![\Delta H^o_{rxn}=[(1\times (-2755))]-[(1\times (-286))+(2\times (-2341))]\\\\\Delta H^o_{rxn}=2213kJ](/tpl/images/0174/3643/044fa.png)


