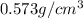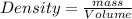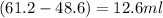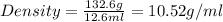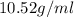Chemistry, 12.08.2019 17:20 jhanley4637
Alarge piece of jewelry has a mass of 132.6 g. a graduated cylinder initially contains 48.6 ml water. when the jewelry is submerged in the graduated cylinder, the total volume increases to 61.2 ml.
(a) determine the density of this piece of jewelry.
(b) assuming that the jewelry is made from only one suvstance, what substance is it likely to be? explain

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2
You know the right answer?
Alarge piece of jewelry has a mass of 132.6 g. a graduated cylinder initially contains 48.6 ml water...
Questions



Mathematics, 19.03.2020 02:33



Social Studies, 19.03.2020 02:33





Mathematics, 19.03.2020 02:33

Mathematics, 19.03.2020 02:33