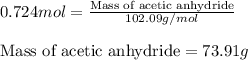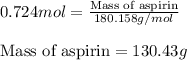Chemistry, 12.08.2019 17:10 silveryflight
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3). the balanced equation isc7h6o3 + c4h6o3 → c9h8o4 + hc2h3o2(a) what mass of acetic anhydride is needed to completely consume 1.00 x 10^2 g salicylic acid? (b) what is the maximum mass of aspirin (the theoretical yield) that could be produced in this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2
You know the right answer?
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3)....
Questions

Chemistry, 28.04.2021 19:10


History, 28.04.2021 19:10

Mathematics, 28.04.2021 19:10

Mathematics, 28.04.2021 19:10




English, 28.04.2021 19:10

Chemistry, 28.04.2021 19:10

Mathematics, 28.04.2021 19:10

Chemistry, 28.04.2021 19:10




English, 28.04.2021 19:10

Mathematics, 28.04.2021 19:10

Mathematics, 28.04.2021 19:10


Computers and Technology, 28.04.2021 19:10

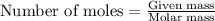 .....(1)
.....(1)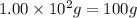
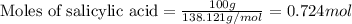
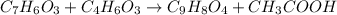
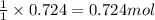 of acetic anhydride.
of acetic anhydride.