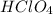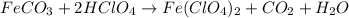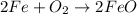Chemistry, 10.08.2019 06:10 briansalazar17
Predict the products of each of the following reactions and then balance the chemical equations.
(a) fe is heated in an atmosphere of steam.
(b) naoh is added to a solution of fe(no3)3.
(c) feso4 is added to an acidic solution of kmno4.
(d) fe is added to a dilute solution of h2so4.
(e) a solution of fe(no3)2 and hno3 is allowed to stand in air.
(f) feco3 is added to a solution of hclo4.
(g) fe is heated in air.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Predict the products of each of the following reactions and then balance the chemical equations.
Questions


Mathematics, 25.08.2021 06:10









Mathematics, 25.08.2021 06:10

Mathematics, 25.08.2021 06:10

English, 25.08.2021 06:10

Chemistry, 25.08.2021 06:10

Chemistry, 25.08.2021 06:10


Mathematics, 25.08.2021 06:10


Mathematics, 25.08.2021 06:10

Mathematics, 25.08.2021 06:10

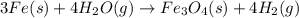
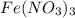
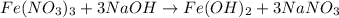
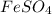 is added to an acidic solution of
is added to an acidic solution of 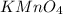
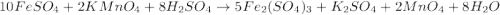
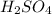
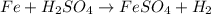
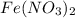 and
and 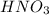 is allowed to stand in air.
is allowed to stand in air.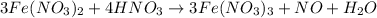
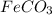 is added to a solution of
is added to a solution of