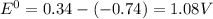Agalvanic (voltaic) cell consists of an electrode composed of chromium in a 1.0 m chromium(iii) ion solution and another electrode composed of copper in a 1.0 m copper(ii) ion solution, connected by a salt bridge. calculate the standard potential for this cell at 25 °c. refer to the list

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

You know the right answer?
Agalvanic (voltaic) cell consists of an electrode composed of chromium in a 1.0 m chromium(iii) ion...
Questions


Mathematics, 31.01.2020 21:51


History, 31.01.2020 21:51


Mathematics, 31.01.2020 21:51

Mathematics, 31.01.2020 21:51




Physics, 31.01.2020 21:51

Mathematics, 31.01.2020 21:51

History, 31.01.2020 21:51



Mathematics, 31.01.2020 21:51

Chemistry, 31.01.2020 21:51


History, 31.01.2020 21:51

Mathematics, 31.01.2020 21:51

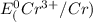 =-0.74V[/tex]
=-0.74V[/tex]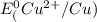 =0.34V[/tex]
=0.34V[/tex]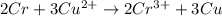
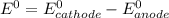
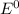 are standard reduction potentials, when concentration is 1M.
are standard reduction potentials, when concentration is 1M.![E^0=E^0_{[Cu^{2+}/Ni]}- E^0_{[Cr^{3+}/Cr]}](/tpl/images/0174/1025/838bc.png)