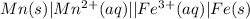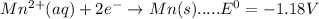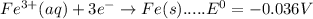Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Anurse practitioner prepares an injection of promethazine, an antihistamine used to treat allergic rhinitis. if the stock bottle is labeled 25 mg/ml and the order is a dose of 11.0 mg , how many milliliters will the nurse draw up in the syringe?
Answers: 3

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1
You know the right answer?
Consider the following half-reactions and their standard reduction potentials then give the standard...
Questions

Social Studies, 23.09.2019 14:10






History, 23.09.2019 14:10

Arts, 23.09.2019 14:10



Biology, 23.09.2019 14:10



Mathematics, 23.09.2019 14:10





Arts, 23.09.2019 14:20