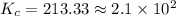Chemistry, 10.08.2019 01:20 mikemurray115
The molar concentrations for the reactants and products at equilibrium are found to be [ccl4]=1.0 m, [o2]=0.3 m, [cocl2]=4.0 m, and [cl2]=2.0 m. what is the value of the equilibrium constant for this reaction? 2ccl4(g)+o2(g)⇌2cocl2(g)+2cl2(g) express your answer numerically using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Perform the following mathematical operations and report the answer to the appropriate number of significant figures 5.87998 + 3.100
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
The molar concentrations for the reactants and products at equilibrium are found to be [ccl4]=1.0 m,...
Questions



Mathematics, 24.10.2021 08:30

SAT, 24.10.2021 08:30

SAT, 24.10.2021 08:30

Physics, 24.10.2021 08:30



Mathematics, 24.10.2021 08:30



Physics, 24.10.2021 08:30



Geography, 24.10.2021 08:30


Engineering, 24.10.2021 08:30

Social Studies, 24.10.2021 08:30


 is the value of the equilibrium constant for this reaction.
is the value of the equilibrium constant for this reaction.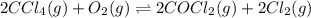
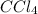 at equilibrium =
at equilibrium =![[CCl_4]=1.0M](/tpl/images/0174/0028/d343b.png)
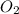 at equilibrium =
at equilibrium =![[O_2]=0.3M](/tpl/images/0174/0028/056b2.png)
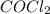 at equilibrium =
at equilibrium =![[COCl_2]=4.0M](/tpl/images/0174/0028/945ea.png)
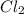 at equilibrium =
at equilibrium =![[Cl_2]=2.0M](/tpl/images/0174/0028/565ad.png)
![K_c=\frac{[COCl_2]^2[Cl_2]^2}{[CCl_4]^2[O_2]}=\frac{(4.0 M)^2\times (2.0M)^2}{(1.0M)^2\times (0.3 M)}](/tpl/images/0174/0028/1316e.png)