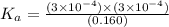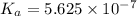Chemistry, 10.08.2019 01:20 Pauline3607
Amonoprotic weak acid, ha, dissociates in water according to the reaction: ha(aq) = h+(aq) + a−(aq). the equilibrium concentrations of the reactants and products are [ha]=0.160 m , [h+]=3.00×10^−4 m , and [a−]=3.00 ×10^−4 m. calculate the value for the acid ha .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 12:50
What is the daughter nucleus produced a. when 217(at) undergoes alpha decay? b. when 103(mo) undergoes beta decay? c. when 188(hg) undergoes positron emission?
Answers: 1

Chemistry, 23.06.2019 14:00
What is the final volume in milliliters when 0.641 l of a 34.0 % (m/v) solution is diluted to 23.5 % (m/v)?
Answers: 1
You know the right answer?
Amonoprotic weak acid, ha, dissociates in water according to the reaction: ha(aq) = h+(aq) + a−(aq)...
Questions

History, 07.07.2019 10:10

History, 07.07.2019 10:10



Chemistry, 07.07.2019 10:10

Biology, 07.07.2019 10:10

Mathematics, 07.07.2019 10:10


Biology, 07.07.2019 10:10





English, 07.07.2019 10:10


History, 07.07.2019 10:10

Mathematics, 07.07.2019 10:10



English, 07.07.2019 10:10

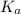 for the acid HA is,
for the acid HA is, 
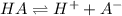
![K_a=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0173/9990/66f51.png)