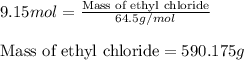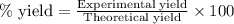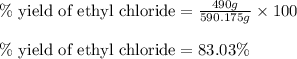Chemistry, 09.08.2019 23:20 4804397217
The reaction of ethane gas (c2h6) with chlorine gas (cl2) produces c2h5cl as its main product. calculate the percent yield of c2h5cl if the reaction of 300 g of ethane with 650 g of chlorine produced 490 g of c2h5cl .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
You know the right answer?
The reaction of ethane gas (c2h6) with chlorine gas (cl2) produces c2h5cl as its main product. calcu...
Questions


Mathematics, 12.02.2021 07:10

Biology, 12.02.2021 07:10

Mathematics, 12.02.2021 07:10

Spanish, 12.02.2021 07:10

Spanish, 12.02.2021 07:10


Mathematics, 12.02.2021 07:10


Mathematics, 12.02.2021 07:10


Chemistry, 12.02.2021 07:10



Mathematics, 12.02.2021 07:10

Mathematics, 12.02.2021 07:10


Mathematics, 12.02.2021 07:10


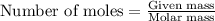 ....(1)
....(1)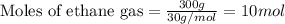
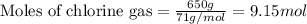
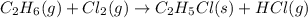
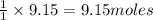 of ethane gas.
of ethane gas.