

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
For the reaction 2 a ⇌ b, the equilibrium concentrations are as follows: [a] = 0.056 mand [b] = 0.1...
Questions

Mathematics, 29.04.2021 01:00

Mathematics, 29.04.2021 01:00

History, 29.04.2021 01:00

History, 29.04.2021 01:00

Mathematics, 29.04.2021 01:00





Mathematics, 29.04.2021 01:00


History, 29.04.2021 01:00



Health, 29.04.2021 01:00

Mathematics, 29.04.2021 01:00

Chemistry, 29.04.2021 01:00

English, 29.04.2021 01:00

Mathematics, 29.04.2021 01:00

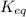 .
.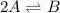
![K_{eq} = \frac{[B]}{[A]^{2}}](/tpl/images/0173/8965/36e57.png)
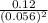
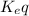 ) for the reaction is 38.
) for the reaction is 38.

